CHM 101: General Chemistry I
Learning Track Courses
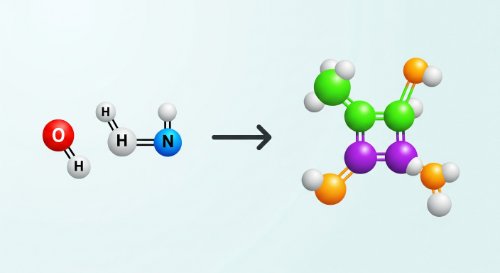
Introduction to Chemistry (Undergraduate Foundation)
Chemistry is the science of matter. This course provides a rigorous introduction to its fundamental principles, moving from the definition of matter and its states to the atomic and molecular structures that govern its behaviour. We will classify matter into elements, compounds, and mixtures, and establish the critical distinction between physical and chemical changes.
A command of chemistry is essential for any quantitative science. The principles covered here are foundational to medicine, engineering, environmental science, and materials development. Understanding how matter is structured and how it transforms is the basis for creating new materials, developing pharmaceuticals, and managing industrial chemical processes safely and efficiently.
Upon completion, you will possess a clear conceptual framework for chemistry. You will be able to classify different types of matter, distinguish between physical and chemical changes, and define elements, compounds, and mixtures. You will also be able to identify the fundamental roles of atoms, molecules, and ions in chemical reactions.
This course is designed for first-year university students beginning their studies in any scientific or engineering discipline. It serves as a mandatory prerequisite for further study in chemistry, chemical engineering, biology, and medicine. It is also suitable for anyone requiring a concise and formal review of foundational chemical concepts.
Introduction to Chemistry (Undergraduate Foundation)
Chemistry is the science of matter. This course provides a rigorous introduction to its fundamental principles, moving from the definition of matter and its states to the atomic and molecular structures that govern its behaviour. We will classify matter into elements, compounds, and mixtures, and establish the critical distinction between physical and chemical changes. A command of chemistry is essential for any quantitative science. The principles covered here are foundational to medicine, engineering, environmental science, and materials development. Understanding how matter is structured and how it transforms is the basis for creating new materials, developing pharmaceuticals, and managing industrial chemical processes safely and efficiently. Upon completion, you will possess a clear conceptual framework for chemistry. You will be able to classify different types of matter, distinguish between physical and chemical changes, and define elements, compounds, and mixtures. You will also be able to identify the fundamental roles of atoms, molecules, and ions in chemical reactions. This course is designed for first-year university students beginning their studies in any scientific or engineering discipline. It serves as a mandatory prerequisite for further study in chemistry, chemical engineering, biology, and medicine. It is also suitable for anyone requiring a concise and formal review of foundational chemical concepts.

Atomic Structure and Periodicity of Elements - Chemistry (Undergraduate Foundation)
This course provides a complete guide to the modern theory of the atom. It traces the historical development of atomic models, from Dalton's foundational theory to the modern understanding of electronic structure. The material covers subatomic particles, the contributions of Thomson and Rutherford, the Bohr model, wave-particle duality, and culminates in a full treatment of electronic configuration and its direct relationship to the periodic trends of the elements.
A command of atomic theory is the absolute foundation of modern chemistry. The electronic structure of the atom dictates all chemical properties and bonding behaviour. This knowledge is essential for understanding the periodic table, predicting chemical reactions, and is the prerequisite for the study of spectroscopy, materials science, and quantum mechanics.
By the end of this course, you will be able to describe the historical evolution of atomic theory. You will also be able to explain the atom's structure, including the roles of protons, neutrons, and electrons. You will determine the electronic configuration of any element and use periodic trends to predict and justify atomic properties like size, ionisation potential, and electronegativity.
This course is for students who have completed an introductory chemistry course. It is a mandatory prerequisite for any student pursuing a degree in chemistry, chemical engineering, materials science, or physics.
Atomic Structure and Periodicity of Elements - Chemistry (Undergraduate Foundation)
This course provides a complete guide to the modern theory of the atom. It traces the historical development of atomic models, from Dalton's foundational theory to the modern understanding of electronic structure. The material covers subatomic particles, the contributions of Thomson and Rutherford, the Bohr model, wave-particle duality, and culminates in a full treatment of electronic configuration and its direct relationship to the periodic trends of the elements. A command of atomic theory is the absolute foundation of modern chemistry. The electronic structure of the atom dictates all chemical properties and bonding behaviour. This knowledge is essential for understanding the periodic table, predicting chemical reactions, and is the prerequisite for the study of spectroscopy, materials science, and quantum mechanics. By the end of this course, you will be able to describe the historical evolution of atomic theory. You will also be able to explain the atom's structure, including the roles of protons, neutrons, and electrons. You will determine the electronic configuration of any element and use periodic trends to predict and justify atomic properties like size, ionisation potential, and electronegativity. This course is for students who have completed an introductory chemistry course. It is a mandatory prerequisite for any student pursuing a degree in chemistry, chemical engineering, materials science, or physics.
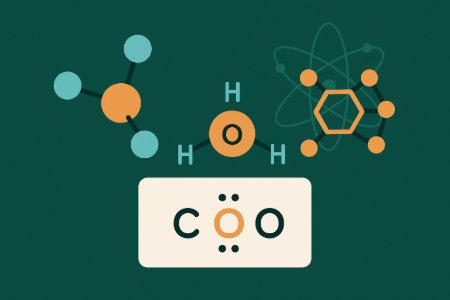
Chemical Bonding and Shapes of Molecules - Chemistry (Undergraduate Foundation)
The shape of a molecule dictates its function. This course provides a practical, foundational treatment of chemical bonding and molecular geometry - the principles governing how atoms connect to form substances. It covers ionic and covalent bonding, the drawing of Lewis structures, and methods for determining molecular shape using VSEPR theory and orbital hybridisation. Finally, we examine intermolecular forces that control a substance's physical properties.
A command of this material is essential for molecular design. Knowledge of bonding and geometry dictates the properties of everything from pharmaceuticals, where shape governs drug-receptor specificity, to new materials like polymers and catalysts. Mastery of this foundational knowledge is the starting point for developing or synthesising any new chemical compound. Our system uses comprehensive videos and allows you to ask questions directly, making it fully online and easy to master at your pace.
By the end of this course, you will be able to draw correct Lewis structures for any molecule, predict its three-dimensional geometry and polarity using VSEPR theory, determine the central atom's hybridisation and bond types, and identify the various intermolecular forces present in a substance.
This course is mandatory for all undergraduate students of chemistry, biochemistry, and materials science, and is a direct prerequisite for studying organic chemistry. It benefits anyone needing a rigorous, structured, student-centred refresher or initial exposure to this critical subject, assuming a complete understanding of atomic theory and electronic configuration.
Chemical Bonding and Shapes of Molecules - Chemistry (Undergraduate Foundation)
The shape of a molecule dictates its function. This course provides a practical, foundational treatment of chemical bonding and molecular geometry - the principles governing how atoms connect to form substances. It covers ionic and covalent bonding, the drawing of Lewis structures, and methods for determining molecular shape using VSEPR theory and orbital hybridisation. Finally, we examine intermolecular forces that control a substance's physical properties. A command of this material is essential for molecular design. Knowledge of bonding and geometry dictates the properties of everything from pharmaceuticals, where shape governs drug-receptor specificity, to new materials like polymers and catalysts. Mastery of this foundational knowledge is the starting point for developing or synthesising any new chemical compound. Our system uses comprehensive videos and allows you to ask questions directly, making it fully online and easy to master at your pace. By the end of this course, you will be able to draw correct Lewis structures for any molecule, predict its three-dimensional geometry and polarity using VSEPR theory, determine the central atom's hybridisation and bond types, and identify the various intermolecular forces present in a substance. This course is mandatory for all undergraduate students of chemistry, biochemistry, and materials science, and is a direct prerequisite for studying organic chemistry. It benefits anyone needing a rigorous, structured, student-centred refresher or initial exposure to this critical subject, assuming a complete understanding of atomic theory and electronic configuration.
Kinetic Theory of Matter and Gas Laws - Chemistry (Undergraduate Foundation)
This course provides a rigorous treatment of the Kinetic Theory and the Gas Laws, explaining the physical principles that control the behaviour of solids, liquids, and gases. We move from the foundational kinetic postulates to the precise mathematical relationships required to model matter's response to energy, pressure, and temperature.
These principles dictate real-world engineering, not just textbook theory. You will apply this knowledge to model atmospheric dynamics, design efficient chemical reactors, and optimise engine performance. A command of these laws allows you to quantify substance behaviour under varying conditions, solving the calculation pain points often faced in process engineering and physical science.
By the end of this course, you will confidently apply Boyle's, Charles's, Avogadro's, and the Combined Gas Laws to determine unknown state variables. You will derive and utilize the Ideal Gas Equation, PV = nRT, and explain gas properties using kinetic theory postulates. Additionally, you will analyse the structure of solids and calculate gas density.
This is a mandatory foundation for Chemistry and Chemical Engineering undergraduates and a prerequisite for thermodynamics. It is intended for learners with a grasp of chemical bonding who require a structured, student-centred alternative to disjointed free resources.
Kinetic Theory of Matter and Gas Laws - Chemistry (Undergraduate Foundation)
This course provides a rigorous treatment of the Kinetic Theory and the Gas Laws, explaining the physical principles that control the behaviour of solids, liquids, and gases. We move from the foundational kinetic postulates to the precise mathematical relationships required to model matter's response to energy, pressure, and temperature. These principles dictate real-world engineering, not just textbook theory. You will apply this knowledge to model atmospheric dynamics, design efficient chemical reactors, and optimise engine performance. A command of these laws allows you to quantify substance behaviour under varying conditions, solving the calculation pain points often faced in process engineering and physical science. By the end of this course, you will confidently apply Boyle's, Charles's, Avogadro's, and the Combined Gas Laws to determine unknown state variables. You will derive and utilize the Ideal Gas Equation, PV = nRT, and explain gas properties using kinetic theory postulates. Additionally, you will analyse the structure of solids and calculate gas density. This is a mandatory foundation for Chemistry and Chemical Engineering undergraduates and a prerequisite for thermodynamics. It is intended for learners with a grasp of chemical bonding who require a structured, student-centred alternative to disjointed free resources.

Stoichiometry of Composition - Chemistry (Undergraduate Foundation)
Grasp the fundamental language of chemistry through Stoichiometry of Composition. This concise undergraduate-foundation course systematically establishes core quantitative concepts essential for all subsequent chemistry studies. We will precisely define and calculate relative atomic mass, master the critical mole concept to transition between the microscopic and macroscopic worlds, determine molar masses, and then apply these principles to calculate percent composition and accurately derive both empirical and molecular formulae from experimental data. The content is structured for immediate application, moving from foundational definitions to complex problem-solving.
Quantitative chemistry is not abstract theory; it is the practical basis for chemical synthesis and analysis across science and industry. Learners will develop the ability to accurately predict reactant and product ratios in chemical reactions, interpret laboratory data for compound identification, and correctly formulate materials in fields such as chemical engineering, pharmaceuticals, and materials science. Mastery of these stoichiometric calculations is non-negotiable for success in laboratory work and industrial scale-up.
Upon completion, you will be able to calculate and interpret relative atomic and molecular masses; confidently apply the mole concept to interconvert mass, moles, and number of particles; calculate the molar mass of any compound; precisely determine the percent composition of a substance; and successfully solve multi-step problems to deduce a compound's empirical and molecular formulae. The course culminates in extensive practice to secure computational fluency.
This course is essential for undergraduate students beginning any science or engineering programme that requires a solid foundation in Chemistry, particularly those preparing for university-level coursework. It also serves as an intensive, structured refresher for advanced students, technical professionals needing to reinforce core computational skills, and anyone using UniDrills to self-study for competitive professional examinations.
Stoichiometry of Composition - Chemistry (Undergraduate Foundation)
Grasp the fundamental language of chemistry through Stoichiometry of Composition. This concise undergraduate-foundation course systematically establishes core quantitative concepts essential for all subsequent chemistry studies. We will precisely define and calculate relative atomic mass, master the critical mole concept to transition between the microscopic and macroscopic worlds, determine molar masses, and then apply these principles to calculate percent composition and accurately derive both empirical and molecular formulae from experimental data. The content is structured for immediate application, moving from foundational definitions to complex problem-solving. Quantitative chemistry is not abstract theory; it is the practical basis for chemical synthesis and analysis across science and industry. Learners will develop the ability to accurately predict reactant and product ratios in chemical reactions, interpret laboratory data for compound identification, and correctly formulate materials in fields such as chemical engineering, pharmaceuticals, and materials science. Mastery of these stoichiometric calculations is non-negotiable for success in laboratory work and industrial scale-up. Upon completion, you will be able to calculate and interpret relative atomic and molecular masses; confidently apply the mole concept to interconvert mass, moles, and number of particles; calculate the molar mass of any compound; precisely determine the percent composition of a substance; and successfully solve multi-step problems to deduce a compound's empirical and molecular formulae. The course culminates in extensive practice to secure computational fluency. This course is essential for undergraduate students beginning any science or engineering programme that requires a solid foundation in Chemistry, particularly those preparing for university-level coursework. It also serves as an intensive, structured refresher for advanced students, technical professionals needing to reinforce core computational skills, and anyone using UniDrills to self-study for competitive professional examinations.
Stoichiometry of Reactions - Chemistry (Undergraduate Foundation)
Master the quantitative prediction of chemical change with Stoichiometry of Reactions. This undergraduate foundation course focuses on transforming conceptual chemical reactions into balanced, calculable equations. We begin by applying the conservation of mass principle to balance equations through inspection, the algebraic method, and the oxidation number change method. Next, we simplify reactions in solution by deriving total and net ionic equations, rigorously defining redox reactions, and teaching the precise assignment of oxidation numbers. The final section culminates in balancing complex redox equations using the half-reaction (electron transfer) method in both acidic and basic media.
Accurate reaction stoichiometry is the cornerstone of chemical processing, environmental analysis, and energy generation. Learners will gain the necessary computational skills to predict theoretical yields, determine limiting reagents in industrial synthesis, and analyse electrochemical processes like batteries and corrosion. Correctly balancing and interpreting chemical equations ensures laboratory safety, validates experimental results, and underpins quantitative decision-making in chemical engineering, materials science, and biochemistry.
After completing this course, you will be proficient in balancing any chemical equation based on the conservation of mass; accurately converting molecular equations into total and net ionic forms; defining and identifying oxidation and reduction processes, including their agents; assigning oxidation numbers to elements in compounds; and confidently balancing complicated redox reactions in both acidic and basic solutions. The course concludes with comprehensive practice problems to cement computational accuracy in reaction stoichiometry.
This course is specifically designed for undergraduate students beginning foundational Chemistry or related programmes, including Chemical Engineering, Pharmacy, and pure Sciences, where proficiency in calculating reaction quantities is mandatory. It is also an ideal, focused resource for pre-university students seeking an advanced start, or for any professional needing a rapid, expert-led review of fundamental reaction stoichiometry principles for accreditation or further study.
Stoichiometry of Reactions - Chemistry (Undergraduate Foundation)
Master the quantitative prediction of chemical change with Stoichiometry of Reactions. This undergraduate foundation course focuses on transforming conceptual chemical reactions into balanced, calculable equations. We begin by applying the conservation of mass principle to balance equations through inspection, the algebraic method, and the oxidation number change method. Next, we simplify reactions in solution by deriving total and net ionic equations, rigorously defining redox reactions, and teaching the precise assignment of oxidation numbers. The final section culminates in balancing complex redox equations using the half-reaction (electron transfer) method in both acidic and basic media. Accurate reaction stoichiometry is the cornerstone of chemical processing, environmental analysis, and energy generation. Learners will gain the necessary computational skills to predict theoretical yields, determine limiting reagents in industrial synthesis, and analyse electrochemical processes like batteries and corrosion. Correctly balancing and interpreting chemical equations ensures laboratory safety, validates experimental results, and underpins quantitative decision-making in chemical engineering, materials science, and biochemistry. After completing this course, you will be proficient in balancing any chemical equation based on the conservation of mass; accurately converting molecular equations into total and net ionic forms; defining and identifying oxidation and reduction processes, including their agents; assigning oxidation numbers to elements in compounds; and confidently balancing complicated redox reactions in both acidic and basic solutions. The course concludes with comprehensive practice problems to cement computational accuracy in reaction stoichiometry. This course is specifically designed for undergraduate students beginning foundational Chemistry or related programmes, including Chemical Engineering, Pharmacy, and pure Sciences, where proficiency in calculating reaction quantities is mandatory. It is also an ideal, focused resource for pre-university students seeking an advanced start, or for any professional needing a rapid, expert-led review of fundamental reaction stoichiometry principles for accreditation or further study.

Stoichiometry of Solutions - Chemistry (Undergraduate Foundation)
Precision in volumetric analysis is the difference between experimental success and catastrophic failure in the laboratory. This course provides a rigorous mathematical framework for quantifying dissolved substances, beginning with fundamental concentration units like molarity and mass concentration before progressing to the principles of dilution and standard solution preparation. You will master the stoichiometric requirements of complex chemical interactions, including precipitation, acid-base neutralisation, and redox reactions, through a series of intensive worked examples covering advanced techniques such as back-titration and standardisation.
Quantitative titration skills are the industrial standard for quality control and analytical research. Proficiency in solution stoichiometry allows for the exact determination of drug purity in pharmaceuticals, contaminant levels in environmental monitoring, and reactant concentrations in industrial chemical synthesis. Mastering these computational methods ensures the accuracy required for professional competency in any clinical or academic laboratory setting.
By the conclusion of this course, you will be able to calculate precise concentrations from raw experimental data across multiple reaction types. You will acquire the technical ability to prepare primary standard solutions, apply dilution laws to reach target molarities, resolve limiting reagent problems in aqueous phases, and execute complex multi-step stoichiometric calculations for redox and back-titration scenarios. These skills are essential for converting laboratory observations into reportable analytical results.
This course is a mandatory requirement for undergraduate students in Chemistry, Pharmacy, Biochemistry, and Chemical Engineering. It also serves as a critical computational review for laboratory technicians and research assistants preparing for technical certification or professional exams. Even for those in peripheral scientific fields, the rigorous logic and mathematical precision developed here provide a necessary foundation for any discipline requiring core analytical data processing.
Stoichiometry of Solutions - Chemistry (Undergraduate Foundation)
Precision in volumetric analysis is the difference between experimental success and catastrophic failure in the laboratory. This course provides a rigorous mathematical framework for quantifying dissolved substances, beginning with fundamental concentration units like molarity and mass concentration before progressing to the principles of dilution and standard solution preparation. You will master the stoichiometric requirements of complex chemical interactions, including precipitation, acid-base neutralisation, and redox reactions, through a series of intensive worked examples covering advanced techniques such as back-titration and standardisation. Quantitative titration skills are the industrial standard for quality control and analytical research. Proficiency in solution stoichiometry allows for the exact determination of drug purity in pharmaceuticals, contaminant levels in environmental monitoring, and reactant concentrations in industrial chemical synthesis. Mastering these computational methods ensures the accuracy required for professional competency in any clinical or academic laboratory setting. By the conclusion of this course, you will be able to calculate precise concentrations from raw experimental data across multiple reaction types. You will acquire the technical ability to prepare primary standard solutions, apply dilution laws to reach target molarities, resolve limiting reagent problems in aqueous phases, and execute complex multi-step stoichiometric calculations for redox and back-titration scenarios. These skills are essential for converting laboratory observations into reportable analytical results. This course is a mandatory requirement for undergraduate students in Chemistry, Pharmacy, Biochemistry, and Chemical Engineering. It also serves as a critical computational review for laboratory technicians and research assistants preparing for technical certification or professional exams. Even for those in peripheral scientific fields, the rigorous logic and mathematical precision developed here provide a necessary foundation for any discipline requiring core analytical data processing.
Chemical Equilibria and Acid-Base Chemistry (Undergraduate Foundation)
This course provides a complete guide to chemical equilibria, the state where the rates of forward and reverse reactions are equal. It covers the law of mass action, the definition and calculation of the equilibrium constant, and the factors that can cause a shift in the equilibrium position. The course then applies these principles to the study of aqueous equilibria, including the properties of acids, bases, and salts, and the calculation of pH.
The principles of equilibrium govern the outcomes of all reversible reactions, from industrial synthesis to biological processes. A command of this topic is essential for chemists and chemical engineers to maximise the yield of a desired product, for environmental scientists to understand natural water systems, and for biochemists to analyse metabolic pathways.
By the end of this course, you will be able to write the expression for the equilibrium constant for any reversible reaction, use Le Chatelier's principle to predict how a system at equilibrium will respond to changes in concentration, pressure, or temperature, and perform calculations involving the pH of acidic and basic solutions.
This course is for students who have a solid foundation in stoichiometry. It is a mandatory course for all students of chemistry and chemical engineering and is a direct prerequisite for the study of analytical chemistry, biochemistry, and environmental science.
Chemical Equilibria and Acid-Base Chemistry (Undergraduate Foundation)
This course provides a complete guide to chemical equilibria, the state where the rates of forward and reverse reactions are equal. It covers the law of mass action, the definition and calculation of the equilibrium constant, and the factors that can cause a shift in the equilibrium position. The course then applies these principles to the study of aqueous equilibria, including the properties of acids, bases, and salts, and the calculation of pH. The principles of equilibrium govern the outcomes of all reversible reactions, from industrial synthesis to biological processes. A command of this topic is essential for chemists and chemical engineers to maximise the yield of a desired product, for environmental scientists to understand natural water systems, and for biochemists to analyse metabolic pathways. By the end of this course, you will be able to write the expression for the equilibrium constant for any reversible reaction, use Le Chatelier's principle to predict how a system at equilibrium will respond to changes in concentration, pressure, or temperature, and perform calculations involving the pH of acidic and basic solutions. This course is for students who have a solid foundation in stoichiometry. It is a mandatory course for all students of chemistry and chemical engineering and is a direct prerequisite for the study of analytical chemistry, biochemistry, and environmental science.
Chemical Thermodynamics - Chemistry (Undergraduate Foundation)
Energy drives every chemical change in our universe. This course explains how heat, work, and internal energy dictate the behaviour of matter from simple phase changes to complex chemical reactions. You will master the laws of thermodynamics, enthalpy calculations, entropy changes, and the ultimate predictor of spontaneity, Gibbs free energy.
These principles are the backbone of modern industry. Chemical engineers use them to design efficient industrial reactors and cooling systems. Materials scientists apply them to develop better batteries and energy sources, while biochemists use these same laws to understand the energy flow within living cells. Mastering these concepts is essential for anyone aiming to solve energy-related problems in the real world.
By the end of this course, you will be able to distinguish between open, closed, and isolated systems and calculate heat changes using specific heat capacities. You will apply Hess’s law and bond energies to find reaction enthalpies and use the second law of thermodynamics to predict disorder through entropy. Finally, you will calculate Gibbs free energy to determine exactly if and when a reaction will occur naturally under various temperatures.
This course is built for undergraduate students in chemistry, chemical engineering, and materials science who need a solid foundation in physical chemistry. It also benefits secondary school leavers preparing for university-level science and professionals looking to refresh their technical knowledge of energy systems. Even those outside these fields will gain a logical framework for understanding how energy and disorder shape the physical world.
Chemical Thermodynamics - Chemistry (Undergraduate Foundation)
Energy drives every chemical change in our universe. This course explains how heat, work, and internal energy dictate the behaviour of matter from simple phase changes to complex chemical reactions. You will master the laws of thermodynamics, enthalpy calculations, entropy changes, and the ultimate predictor of spontaneity, Gibbs free energy. These principles are the backbone of modern industry. Chemical engineers use them to design efficient industrial reactors and cooling systems. Materials scientists apply them to develop better batteries and energy sources, while biochemists use these same laws to understand the energy flow within living cells. Mastering these concepts is essential for anyone aiming to solve energy-related problems in the real world. By the end of this course, you will be able to distinguish between open, closed, and isolated systems and calculate heat changes using specific heat capacities. You will apply Hess’s law and bond energies to find reaction enthalpies and use the second law of thermodynamics to predict disorder through entropy. Finally, you will calculate Gibbs free energy to determine exactly if and when a reaction will occur naturally under various temperatures. This course is built for undergraduate students in chemistry, chemical engineering, and materials science who need a solid foundation in physical chemistry. It also benefits secondary school leavers preparing for university-level science and professionals looking to refresh their technical knowledge of energy systems. Even those outside these fields will gain a logical framework for understanding how energy and disorder shape the physical world.
Chemical Kinetics - Chemistry (Undergraduate Foundation)
This course provides a complete guide to chemical kinetics, the study of the rates and mechanisms of chemical reactions. It covers the formal definition of reaction rate, the concept of reaction order, and the determination of rate laws from experimental data. The material also introduces the collision theory, the role of activation energy, and the Arrhenius equation.
Chemical kinetics is essential for controlling chemical reactions in industrial manufacturing, pharmaceutical development, and environmental science. The principles are used to optimise production yields by speeding up desired reactions, to develop catalysts that lower energy consumption, and to understand the complex reaction pathways in atmospheric and biological systems.
By the end of this course, you will be able to define the rate of a reaction and determine the rate law and rate constant from experimental data. You will also be able to use the concept of activation energy to explain the effect of temperature on reaction rate and understand the basic principles of reaction mechanisms.
This course is for students who have a solid foundation in stoichiometry and chemical equilibria. It is a mandatory course for all students of chemistry and chemical engineering and is a direct prerequisite for the study of physical chemistry, industrial chemistry, and biochemistry.
Chemical Kinetics - Chemistry (Undergraduate Foundation)
This course provides a complete guide to chemical kinetics, the study of the rates and mechanisms of chemical reactions. It covers the formal definition of reaction rate, the concept of reaction order, and the determination of rate laws from experimental data. The material also introduces the collision theory, the role of activation energy, and the Arrhenius equation. Chemical kinetics is essential for controlling chemical reactions in industrial manufacturing, pharmaceutical development, and environmental science. The principles are used to optimise production yields by speeding up desired reactions, to develop catalysts that lower energy consumption, and to understand the complex reaction pathways in atmospheric and biological systems. By the end of this course, you will be able to define the rate of a reaction and determine the rate law and rate constant from experimental data. You will also be able to use the concept of activation energy to explain the effect of temperature on reaction rate and understand the basic principles of reaction mechanisms. This course is for students who have a solid foundation in stoichiometry and chemical equilibria. It is a mandatory course for all students of chemistry and chemical engineering and is a direct prerequisite for the study of physical chemistry, industrial chemistry, and biochemistry.
Electrochemistry (Undergraduate Foundation)
This course provides a complete guide to electrochemistry, the study of the relationship between chemical reactions and electrical energy. It covers the principles of oxidation-reduction (redox) reactions and their application in electrochemical cells. The material details the structure and function of both galvanic (voltaic) cells, which produce electricity, and electrolytic cells, which use electricity to drive non-spontaneous reactions.
Electrochemistry is the science behind all modern portable energy storage and industrial metal production. The principles covered are the basis for batteries, fuel cells, and rechargeable power sources. This knowledge is also critical for understanding corrosion, electroplating, and the industrial-scale electrolysis used to produce fundamental materials like aluminium and chlorine.
By the end of this course, you will be able to identify the components of any electrochemical cell and predict the direction of electron flow. You will also be able to calculate standard cell potentials using a table of standard reduction potentials, apply Faraday's laws to electrolytic calculations, and use the Nernst equation to determine cell potential under non-standard conditions.
This course is for students who have a solid foundation in stoichiometry and redox reactions. It is a mandatory course for all students of chemistry and chemical engineering and is a direct prerequisite for the study of analytical chemistry, materials science, and electrical engineering.
Electrochemistry (Undergraduate Foundation)
This course provides a complete guide to electrochemistry, the study of the relationship between chemical reactions and electrical energy. It covers the principles of oxidation-reduction (redox) reactions and their application in electrochemical cells. The material details the structure and function of both galvanic (voltaic) cells, which produce electricity, and electrolytic cells, which use electricity to drive non-spontaneous reactions. Electrochemistry is the science behind all modern portable energy storage and industrial metal production. The principles covered are the basis for batteries, fuel cells, and rechargeable power sources. This knowledge is also critical for understanding corrosion, electroplating, and the industrial-scale electrolysis used to produce fundamental materials like aluminium and chlorine. By the end of this course, you will be able to identify the components of any electrochemical cell and predict the direction of electron flow. You will also be able to calculate standard cell potentials using a table of standard reduction potentials, apply Faraday's laws to electrolytic calculations, and use the Nernst equation to determine cell potential under non-standard conditions. This course is for students who have a solid foundation in stoichiometry and redox reactions. It is a mandatory course for all students of chemistry and chemical engineering and is a direct prerequisite for the study of analytical chemistry, materials science, and electrical engineering.
Radioactivity - Chemistry (Undergraduate Foundation)
This course provides a complete introduction to radioactivity and nuclear chemistry. It covers the principles of radioactive disintegration, the different types of decay, and the concept of half-life. The material also introduces the powerful processes of nuclear fission and fusion, and explores the practical uses of radioisotopes.
An understanding of radioactivity is essential in the modern world. These principles are the foundation of nuclear power generation, medical imaging techniques like PET scans, and carbon dating in archaeology and geology. This knowledge is critical for applications in medicine, energy production, and environmental science.
By the end of this course, you will be able to describe the process of radioactive disintegration and identify the different types of nuclear radiation. You will also be able to explain the concepts of nuclear fission and fusion and describe the key applications of radioisotopes in medicine and industry.
This course is for students who have a solid foundation in atomic theory. It is a mandatory course for any student of nuclear engineering or health physics and provides essential knowledge for students of chemistry, physics, and medicine.
Radioactivity - Chemistry (Undergraduate Foundation)
This course provides a complete introduction to radioactivity and nuclear chemistry. It covers the principles of radioactive disintegration, the different types of decay, and the concept of half-life. The material also introduces the powerful processes of nuclear fission and fusion, and explores the practical uses of radioisotopes. An understanding of radioactivity is essential in the modern world. These principles are the foundation of nuclear power generation, medical imaging techniques like PET scans, and carbon dating in archaeology and geology. This knowledge is critical for applications in medicine, energy production, and environmental science. By the end of this course, you will be able to describe the process of radioactive disintegration and identify the different types of nuclear radiation. You will also be able to explain the concepts of nuclear fission and fusion and describe the key applications of radioisotopes in medicine and industry. This course is for students who have a solid foundation in atomic theory. It is a mandatory course for any student of nuclear engineering or health physics and provides essential knowledge for students of chemistry, physics, and medicine.